Software article - JTrack-EMA+ development and usability
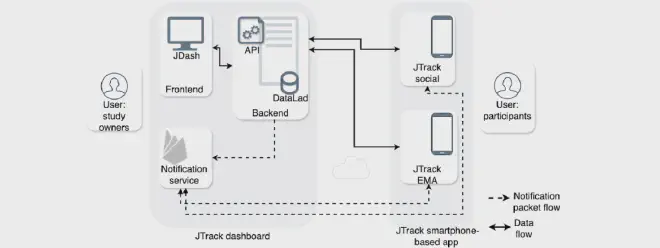
Researchers from ABCD-J sites, Research Centre Jülich and Heinrich-Heine-University, introduce JTrack-EMA+, a mobile health app designed to simplify the deployment of ecological momentary assessments (EMA) via smartphones. Developed with Flutter, the system ensures consistent cross-platform performance, addressing the cost and development challenges in digital health research associated with diverse operating systems.
JTrack-EMA+ consists of:
- A cross-platform mobile app for collecting real-time responses to EMA prompts
- A secure web-based study management dashboard (JDash) for researchers to manage participants, configure studies, monitor compliance, and send push notifications
- Full customization using a JSON file, supporting a wide range of assessment formats and logic to facilitate versatile study design and reusability
The system is designed to comply with General Data Protection Regulation (GDPR) and FAIR data principles, with backend infrastructure ensuring secure storage and anonymized and pseudonymized data collection. In addition, DataLad is implemented as the data management infrastructure to provide data versioning, metadata handling, structured formatting and change tracking.
In a pilot study with 179 parent-newborn pairs followed for 480 days, the JTrack-EMA+ app was deployed and demonstrated practical feasibility of the platform in real-world settings. Participants installed the JTrack app on their mobile phones and responded to weekly assessments of children’s physical and mental development. Among a subgroup of 65 participants who completed assessments for at least 6 months, compliance was initially high with a 66.7% response rate in the first month. Compliance gradually decreased to 42.0% by month six, with a mean overall compliance of 49.3% in the six-month period. Despite a statistically significant decline in compliance over time, the pilot study confirms that JTrack-EMA+ can track individual day-to-day variability over time with a higher compliance rate than employing traditional paper-based EMA.
Overall, the JTrack-EMA+ app and associated study and data management solutions that are being developed as part of the ABCD-J software stack uniquely provide a cross-platform, open-source system that can make mobile health research more accessible, scalable, and secure.
Source: Sahandi Far et al., 2025; www.jmir.org/2025/1/e51689/
There's no articles to list here yet.